What is Atomic Absorption Spectroscopy(AAS)?
The principle of Atomic Absorption Spectroscopy(AAS) is the conversion of the sample to be analysed into atomic vapor state followed by measurement of the absorption at a selected wavelength (characteristic of each element). The concentration of the sample is proportional to the measured absorption. Thus, in AAS, the element is reduced to the elemental state, which is then vaporized and injected in the radiation beam from a suitable source. The various components of atomic absorption spectophotometer are depicted in given figure below.
In atomic absorption spectrometer, a hollow cathode lamp is the source of radiation. Such lamps are now commonly available for all elements that can be analysed. The cathode tube is filled with an inert gas like argon under pressure. Other components include an anode and a hallow cathode. The inside of the cathode is coated with a thin film of the metal to be analysed. On the application of high potential across its electrodes, argon gas inside the cathode gets converted with Ar+ ions due to electric current of several milliamperes which flows through the lamp. The At ions produced attack on the surface of the metal coating of the cathode resulting in sputtering to the metal ions.
The radiation emitted from metal atoms are made to pass through a flame (in the burner) into which the sample to be analysed is injected by an atomizer. In the flame, the metallic component decompose resulting in the formation of a cloy to atoms (which are now in the elemental state). The radiation in flame is partly absorbed by the cloy of atoms; this decreases the radiant energy in the radiation. Finally in resultant radiant energy passes to a monochromator and finally to the detector.
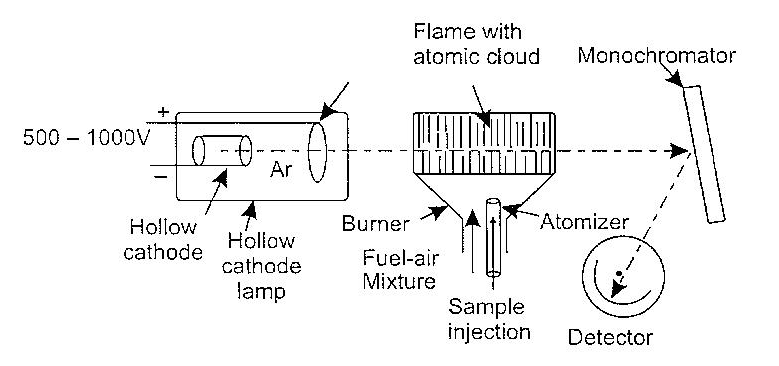
Atomic absorption spectrophotometer showing its various components
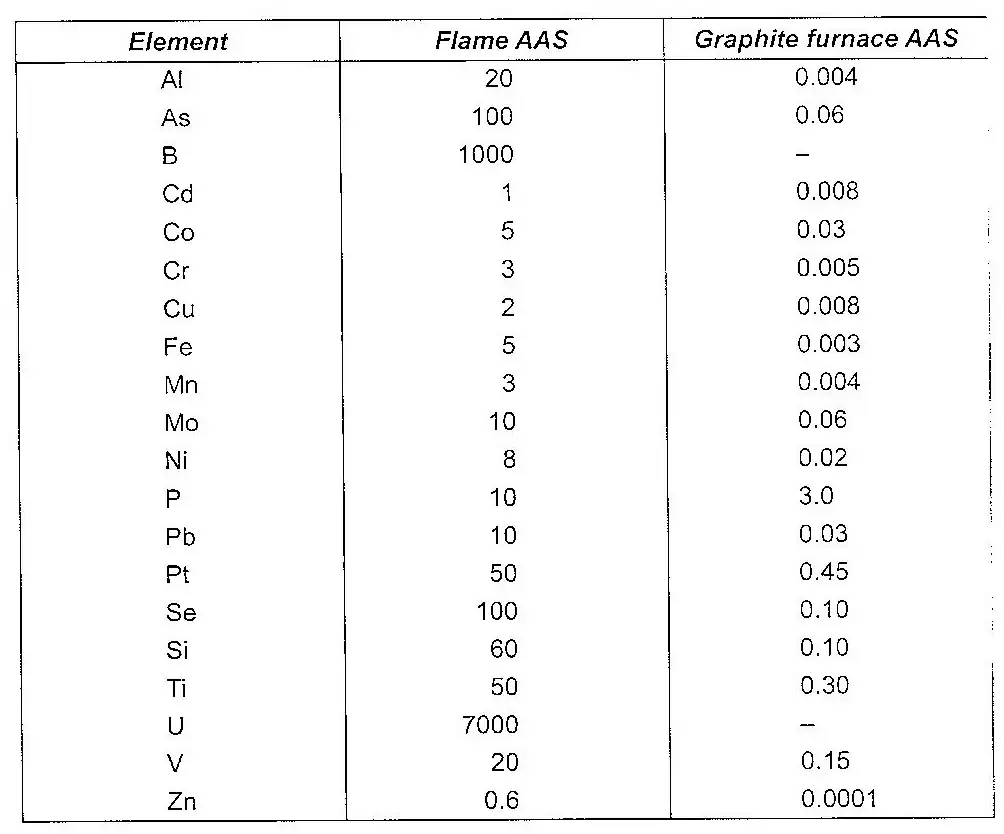
The hollow cathode tubes are now available for all elements that can be determined by an atomic absorption spectrometer. A modification of the AAS is the replacement it an atomizer and the sample holder, for some elements, by a graphite furnace. The detection limits with a graphite furnace are 1000 times better than those from a conventional flame. The table below gives the comparative limits of various metallic pollutants as determined by Flame AAS and graphite furnace AAS.