Depletion of Ozone Hole.
The ozone layer protects life on earth by absorbing the high energy ultraviolet radiations from the sun reaching the earth. These UV radiations can cause skin cancer and also damage the crop plants. In 1987, the thinning of the ozone layer was first observed over the Antarctic’s, which is known as Ozone hole. The following can cause the thinning or the destruction of the ozone layer or the depletion of the ozone layer:
The oxides of nitrogen present in the atmosphere decompose ozone into oxygen:
NO + O3_→ NO2 + O2
NO2 + O → NO + O2
We, thus, see that the oxides of nitrogen (which are formed during thunderstorm and lightening by combination of atmospheric nitrogen and oxygen) destroy or deplete the ozone layer. It is believed that the above process destroys more than 60% of ozone in the atmosphere.
Various nuclear tests conducted in various parts of the world are also responsible for the destruction of ozone layer. Since nuclear tests produce such a high temperature that the atmospheric nitrogen and oxygen combine forming nitric oxide, which as stated above, is responsible for destroying ozone.
Depletion of ozone layer by chlorofluorocarbons (CFC’s)—As the name indicates, chlorofluorocarbons contain carbon, chlorine and fluorine. The CFC’s are non-flammable and non-toxic. They are used as refrigerants (coolants for refrigerators and air conditioners), for making polystyrene, for fire-fighting (special types of CFC’s, known as halons are used as fire extinguishers halons are bromine containing derivatives of chlorofluorocarbons), as propellant for aerosol sprays and as blowing agents for foam plastics.
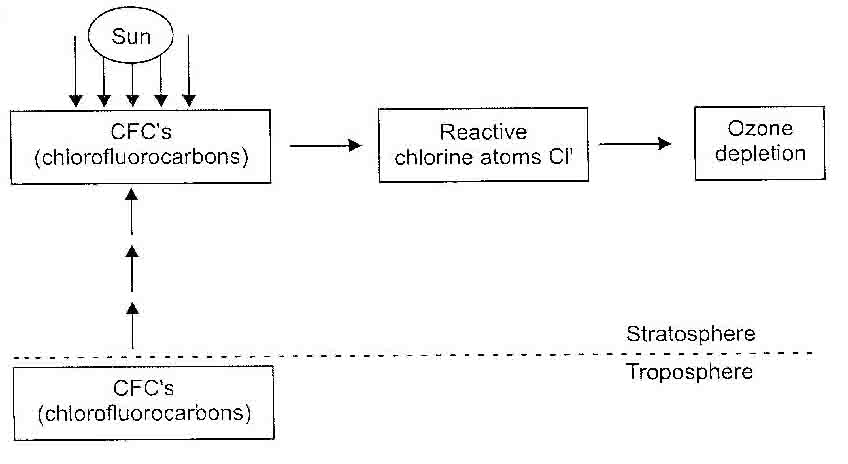
Though unreactive, the CFC’s are a major factor for ozone depletion. Being inert, CFC’s do not easily degrade in the troposphere and so they find their way into the stratosphore which contains the ozone layer. In stratosphore, the CFC’s are photochemically dissociated into reactive chlorine atoms.
It is estimated that for every reactive chlorine atom generated in the stratosphere 1,00,000 molecules of ozone are depleted. The depletion of ozone by chlorofluorocarbons can be schematically represented below in Figure below
Concerned about the ozone hole (which permits harmful UV radiations to reach the earth), a protocol was signed at Montreal, Canada in 1987 in which representative of a number of developed countries reached an agreement to limit the production of CFC’s. The target set was to reduce the use of these harmful CFC’s by 50% by 1998. It was found that the ozone depletion caused by CFC’s was much more alarming. In view of this, the Montreal convention was followed by a conference held in London in 1990 in which many more countries participated. The aim of the Montreal Protocol was affirmed and it was decided to speed up the phasing out of CFC’s.
Prevention or Minimization of Ozone Depletion
As already discussed ozone depletion is effected by oxides of nitrogen and CFC’s. The oxides of nitrogen find their way into the atmosphere by the combination of atmospheric nitrogen and oxygen particularly during thunderstorm and lightening. There is virtually no way to prevent this mode of ozone depletion. However, using alternative substitutes can control the depletion of ozone by CFC’s.
Due to inert nature and enormous stability of CFC’s, they stay in the atmosphere for very long time. For example, the two most commonly used CFC’s viz. CFC-11 (CCI3F) and CFC-12 (CCI2F2) stay in the atmosphere for 75 and 110 years, respectively.
The implies that even if the use of CFC’s is stopped, the already discharged CFC’s in the atmosphere will continue to deplete the ozone for a considerable length of time. In view of this, it is more or less essential to phase out the use of CFC’s and their substitutes must be discovered. The substitutes should have zero potential of ozone depletion and their green house potential should also be very low. It has already been stated that CFC’s are the constituents of green house gases and contribute to global warming.
The following CFC’s are most likely to replace the usual CFC’s. These are HCFC- 123 (CHCI2CF3) and HFC— 134 a (CH2FCF3). These have very low ozone depletion potential and their green house potentials are also quite low. However, their degradation products are toxic in nature.
It should be mentioned at this stage that certain related chemicals like carbon tetrachloride (CCI4) and trichloroethane (CH3CCI3) also have ozone depleting potential. Use of these chemicals also has to be phased out. At present, there are no substitutes for these (trichloroethane is used as a solvent and carbon tetrachloride is used as a raw material for the manufacture of CFC’s and pesticides and as a fire extinguisher).